On the shores of Lake Michigan, one of the largest sources of fresh water in the world, Rebecca Klaper is busy pouring the tiniest materials humans have ever made into tanks containing some of the smallest members of the Great Lakes food web.
Klaper is a professor in the University of Wisconsin–Milwaukee’s School of Freshwater Sciences and a researcher at the Center for Sustainable Nanotechnology, a National Science Foundation-funded collaboration of scientists across the country. And nanomaterials—which she’s pouring into the tanks of tiny zooplankton called daphnia—are almost indescribably small.
A nanometer is one-billionth of a meter. That’s the length your fingernail grows in one second, or 100,000 times smaller than the edge of a piece of paper, or any number of other impossible-sounding comparisons. To qualify as a nanoparticle, a material has to measure between one and 100 nanometers. Forget invisible to the naked eye—a nanoparticle is visible only when using an electron microscope.
But don’t let their size fool you—nanomaterials are a big deal. They’re currently used all over the world to help with everything from building better batteries to improving cancer treatments to fighting foot odor. This means these materials are also constantly shed into our environment, including our freshwater systems, such as when nanoparticle-enhanced sunscreen washes off a swimmer in Lake Michigan.
The problem is, no one’s quite sure what happens when these more-micro-than-microscopic bits of human industry reach the water. And that gets at the heart of Klaper’s research. She wants to understand the fundamental science of how nanomaterials interact with freshwater organisms. If these interactions prove harmful, she wants to understand why and how that happens so that safer, better nanomaterials can be crafted to replace the problematic ones.
“Part of my research is basically preventing something from happening to the Great Lakes,” says Klaper, who’s also director of UWM’s Great Lakes Genomics Center. “We’re trying to create a technology that is beneficial but environmentally safe at the same time.”
Making Things Safer
Klaper didn’t set out to study nanotechnology. Her PhD in ecology is from the University of Georgia, where she worked to understand how natural chemicals in plants interact with insect populations. But in 2001, she moved to Washington, DC, to serve two years as an environmental science and technology policy fellow at the Environmental Protection Agency.
During the fellowship, as Klaper explored how chemicals can alter gene expression and an organism’s DNA, she met researchers working on nanomaterials. That’s when she began to realize the power of working with manmade materials. Unlike naturally occurring chemicals, manmade materials could be designed, engineered, and regulated, potentially leading to better results for both the environment and human health.
Focusing on nanomaterials kept her asking “the same kinds of questions,” Klaper says, “just with different outcomes.” It also put her at the forefront of scientists doing ecological studies of nanomaterials in the environment. After she arrived at UWM’s School of Freshwater Sciences in 2003, the impact of nanomaterials on aquatic ecosystems became a focal point for her research.
Today, Klaper is essentially exploring the idea of building safer chemicals. If scientists can better understand the structure, chemistry, and impact of nanomaterials, they can continue finding uses for them in industry, medicine, and agriculture. But beyond that, scientists can also make sure that, when nanomaterials inevitably end up outside of their intended uses, they do the least amount of harm.
In explaining this intention, Klaper alludes to a cautionary tale about a once-popular material.
In the second half of the 19th century, the industrialized world fell in love with a set of naturally occurring silicate minerals known for their thin, fibrous crystals. Mining of the minerals took off in the United States, Canada, Italy, and South Africa, as the substance’s heat-resistant powers led to widespread use as coatings and insulation, especially in the construction industry.
There was only one problem—asbestos ended up showing a propensity to cause cancer and kill people.
It turns out that what we couldn’t see could hurt us. The tiny, sharp crystals were prone to becoming airborne during mining and manufacturing. By the early 1900s, studies began linking exposure to asbestos to scarring of the lungs and, in the worst cases, death. As the health risks of asbestos became known, countries regulated and, in many cases, banned its use.
The desire to avoid a repeat of all that, Klaper says, drives her work with the Center for Sustainable Nanotechnology.
“One of the exciting things about our initiative,” she says, “is that the federal government decided to make investments in research on the environmental health and safety of nanomaterials along with investing in the growth of the technology.” It’s a proactive approach to developing new technologies that is far different from the business-as-usual story of many past technologies.
So Klaper hopes to help write a new narrative—one about working proactively to identify the dangers of a new technology, and then using that knowledge to build less harmful materials.
For her, that means designing experiments that closely approximate what real-life interactions between nanomaterials and aquatic organisms are likely to be.
A Growing Concern
Since scientists first unlocked the vast potential of very tiny things in the early 1980s, the field of nanotechnology has grown tremendously. Over the last decade or so, nanomaterials have popped up in products across the globe.
Some applications don’t exactly address urgent medical or industrial needs. For example, sometimes nanoparticles of titanium dioxide are added to the powdered sugar that is sprinkled on doughnuts to give them a dense, snow-white coating. Likewise, many brands of athletic socks contain synthetic yarn embedded with nanosilver to discourage the growth of bacteria that cause foot odor.
But other nanomaterials have the potential to powerfully affect human health. Titanium dioxide and zinc oxide nanomaterials increase the effectiveness of sunscreens. And “nanogold,” essentially gold nanoparticles, shows promise as a cancer-targeting tool. These nanoparticles can be injected into the bloodstream, where they are carried to cancerous cells and begin accumulating inside tumors. When the nanogold is then hit with light waves from the edge of the infrared spectrum, it heats up, thereby killing the cancerous cells.
On the green technology front, nanomaterials derived from complex metal oxides, like cobalt or manganese, are providing important advances in next-generation batteries and energy storage devices. Such applications hold the promise of making renewable energy and electric cars more efficient and could push these technologies and products into more widespread use.
But these metal oxides are highly reactive and known to be toxic in their larger forms, which gives some people pause. The truth is that scientists simply don’t know what a lot of the nanomaterials we’re currently manufacturing are going to do once they are out in the world. While nanosilver-enhanced socks might be useful in the fight against foot odor, substantial amounts of these nanoparticles wash out during laundering and pass through the wastewater treatment process, raising concerns about how this antibacterial material will affect freshwater organisms.
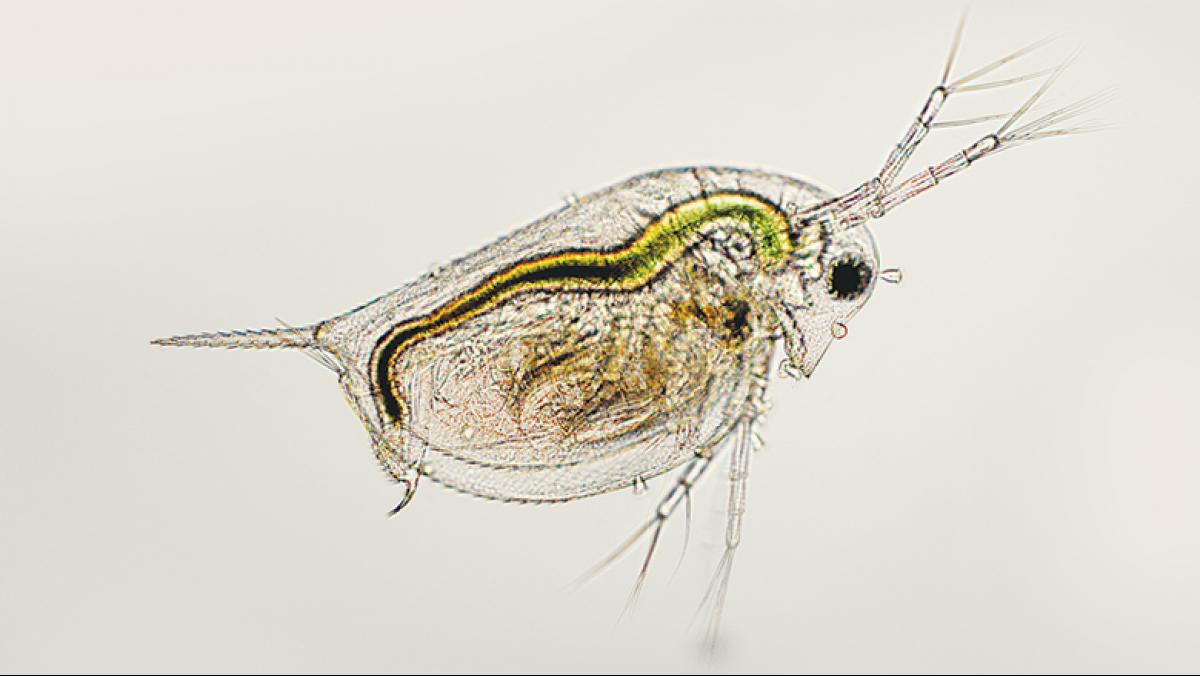
This is why, back in Klaper’s lab, she introduces a minuscule amount of nanomaterial into the aquariums of daphnia zooplankton. Daphnia are tiny, free-floating crustaceans that serve as a foundation for many aquatic food webs. Big fish eat little fish. Little fish eat daphnia. Daphnia eat algae and, it turns out, nanomaterials.
In traditional studies of safe levels for chemical exposure, an organism in a lab is given a dose of a potentially toxic substance, and the amount is increased until obvious impacts occur, such as deformity or death. But, Klaper says, those “dose ’em and kill ’em” experiments provide information on only the most toxic compounds. When it comes to nanoparticles, Klaper says she’s more interested in “the realistic environmental scenario [such as], what kinds of changes happen in the organism as a result of very low exposure over a long period of time?”
For Klaper’s lab, this “long period of time” is often only a month or so—but it is a lifetime for daphnia. “Our goal is to look from development all the way through reproduction, and sometimes we even follow their offspring to reproduction, to see if there are any multigenerational impacts,” Klaper says.
Time Will Tell
Klaper has found that most of the aquatic organisms she studies end up with nanomaterials in their bodies, either by ingesting them or absorbing them through gill tissue. Often, there are no immediate impacts from nanoparticle exposure, but tiny changes to an animal’s inner workings can lead to big repercussions over time.
For example, in previous studies, Klaper has documented gene expression changes related to stress in daphnia exposed to titanium dioxide. In other cases, nanomaterials altered daphnia movement and behavior, which, in the wild, would make them more susceptible to predation and reproductive decline.
Klaper is also looking to see how these nanoparticles affect the life cycle of chironomids. Small, aquatic invertebrates that live in the sediment of lakes and rivers during their larval stage, chironomids swim to the surface as adults to emerge as swarms of midges. The flying insects serve a purpose beyond annoying beachgoers, however. Midges are important to the food webs of fish in the water and of birds on near the shore.
“Nanomaterials can impact the emergence of those midges,” Klaper says. “They can cause them to be smaller or change the amount of eggs they are able to produce, and even their ability to survive and emerge from the water.”
But, Klaper is quick to note, harm to an organism isn’t the only, nor perhaps even the most common, story. Different organisms respond in different ways to different nanomaterials, which leaves Klaper’s lab with the job of understanding why.
Although currently surrounded by more questions than answers, Klaper says, “the good news is that there are quite a few nanoparticles where we don’t find a lot of toxicity. Not all nanoparticles are created equal.” Many nanomaterials are derived from naturally occurring materials, and they appear to be harmless to living organisms.
As scientists in industry work to find new, exciting uses for nanoparticles, Klaper and her colleagues partner with them to ensure the particles are also engineered with the environment in mind. If what we don’t know could hurt us, what Klaper and her colleagues have learned can be used to make us safer.
For example, scientists with the Center for Sustainable Nanotechnology are already changing the chemical structure and physical properties of some metal oxide nanoparticles used in batteries, and they’re also tweaking the delivery method of gold nanoparticles to make them less toxic if they make their way into the environment.
These are small but promising steps, Klaper says, toward the goal of responsibly guiding the development of nanotechnology.
“We want to create materials so that, if they are released, they just don’t have an impact,” she says. “They just sit out there—safe by design.”
This article first appeared in UWM Research, published by the University of Wisconsin–Milwaukee, 2019.